The science behind Disperazol
We have developed Disperazol® as a new, urgently needed weapon in the battle against antimicrobial resistance.
The need to beat biofilms
Biofilms are one of the most difficult to treat and least understood contributors to severe and recurrent bacterial infection and the subsequent development of antimicrobial resistance.
The biofilm life mode refers to the ability of bacteria to aggregate, collectively adhere to a surface and then create a surrounding protective layer around themselves. This is an effective mechanism that protects the bacteria from the killing action of our existing armoury of broad-spectrum antibiotics that were designed to kill free-moving, planktonic bacteria.
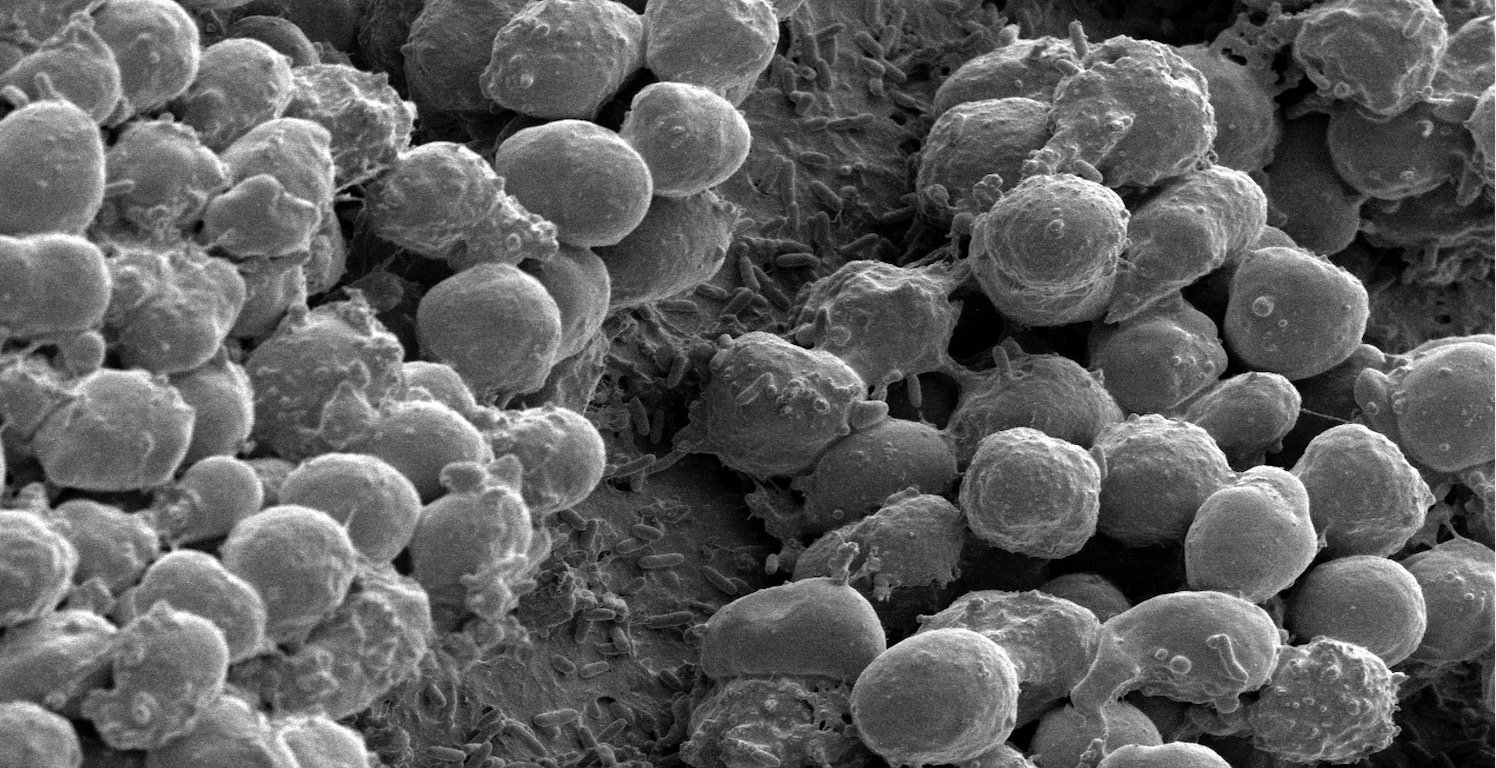
P.aeruginosa biofilm formed on an implant placed in the abdominal cavity of a mouse. The biofilm bacteria are covered by multiple neutrophils of the immune system.
Biofilm-encapsulated bacteria are shielded from the direct effects of existing antibiotics by this protective layer. Once exposed to the specific killing mechanisms of these antibiotics, these bacteria often subsequently mutate to bypass these mechanisms and become resistant to these antibiotics.
Pseudomonas aeruginosa is one of the most prolific of the bacterial biofilm formers and is associated with high levels of resistance to existing antibiotics, high levels of disease burden and high economic cost. It is frequently the cause of respiratory infections, urinary tract infections, wound infections and catheter-associated hospital-acquired infections (HAIs).
P.aeruginosa is ranked as a critical global priority pathogen by the WHO on the basis of its high levels of resistance to existing antibiotics. Together with the lack of new antibiotics in the global development pipeline, this marks a significant gap in available and effective treatments for P.aeruginosa infections.
Disperazol®’s combined novel mechanism of action and novel target make it an effective and potent narrow-spectrum agent against P.aeruginosa when acting in combination with standard of care antibiotics.
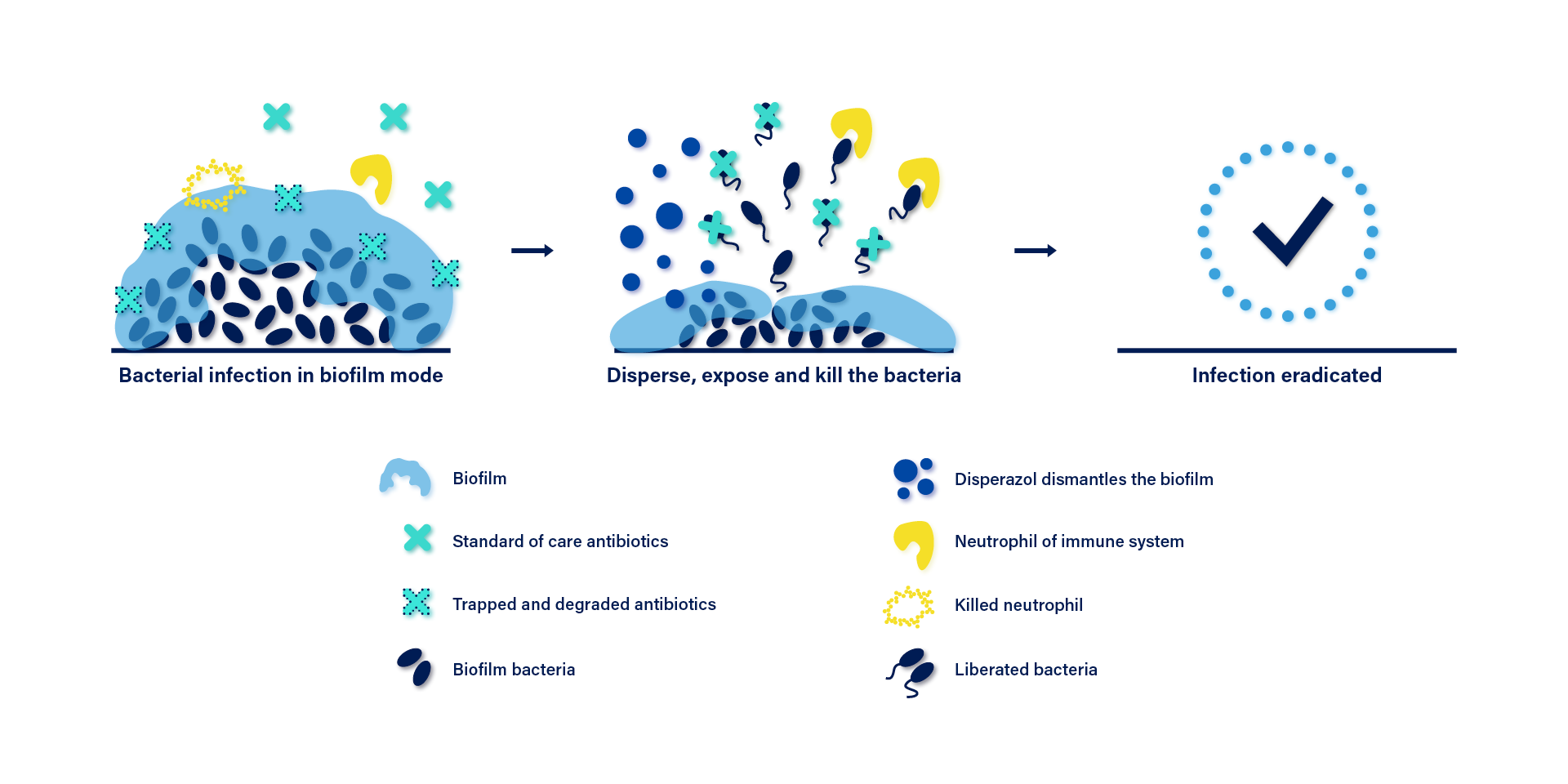
Disperazol® dismantles P.aeruginosa biofilms
Disperazol® is a patent-pending drug that specifically controls the biofilm life cycle of P.aeruginosa. It targets the general and key bacterial process of c-di-GMP signaling that controls the biofilm life cycle.
A high level of c-di-GMP drives planktonic bacteria to form biofilms, whereas reduced c-di-GMP levels promote dispersal of biofilm bacteria. This dispersal causes bacteria to assume the planktonic mode of life, thereby rendering them susceptible to current antibiotics. Disperazol® stimulates the activity of a phosphodiesterase (BifA) which degrades c-di-GMP in P.aeruginosa, resulting in the bacteria losing its protective exopolysaccharide shield and becoming susceptible to be killed by current antibiotics.
Disperazol® activates the PDE denoted BifA to significantly reduce the internal c-di-GMP levels. This signals the bacteria to disperse from the biofilm. The liberated bacteria can be killed by antibiotics and immune cells.